1. Features: High reliability and stability, low electromagnetic interference, and strong anti – interference capability are required to ensure the accuracy and safety of medical devices. Strict requirements for production environment and processes are also needed to ensure the sterility and cleanliness of the products.
2. Applications: Widely used in medical diagnostic equipment.
3. Selling points:
① Compliance with ISO 13485.
② Strict production environment control like cleanrooms.
4. Processes: AOI test, FT test, ICT test, BGA technic, selective wave soldering, SMT, DIP, wave soldering, reflow soldering.
5. Customization: customization according to customers' design files.
6. PCB standards for the medical industry
IPC-A-600: This standard, published by the Association Connecting Electronics Industries (IPC), outlines the acceptability of PCBs regarding surface conditions, hole quality, conductive patterns, and other critical aspects.
IPC-A-610: Also published by IPC, this standard provides criteria for the acceptance of PCB assemblies, ensuring that they meet the necessary workmanship standards for medical devices.
ISO 13485: Although primarily focused on quality management systems, ISO 13485 also covers aspects related to PCB design, manufacturing, and control for medical devices.
IEC 60601-1: This standard applies to medical electrical equipment and systems, specifying safety and performance requirements that PCBs in medical devices must adhere to.
IEC 61010-1: This standard specifically addresses safety requirements for electrical equipment for measurement, control, and laboratory use, which includes many medical devices with PCBs.
UL 60601-1: UL certification ensures that the PCBs and electrical components used in medical devices meet safety and performance standards as required by UL.
RoHS (Restriction of Hazardous Substances) Directive: While not directly a PCB standard, RoHS compliance restricts the use of hazardous substances in PCBs, making them safer for medical device applications.
IEC 62368-1: This standard applies to electrical and electronic equipment within specific voltage ranges and ensures safety requirements for PCBs used in medical devices.
J-STD-001: This standard, also published by IPC, defines the requirements for the soldering of electrical and electronic assemblies, including medical PCBs.
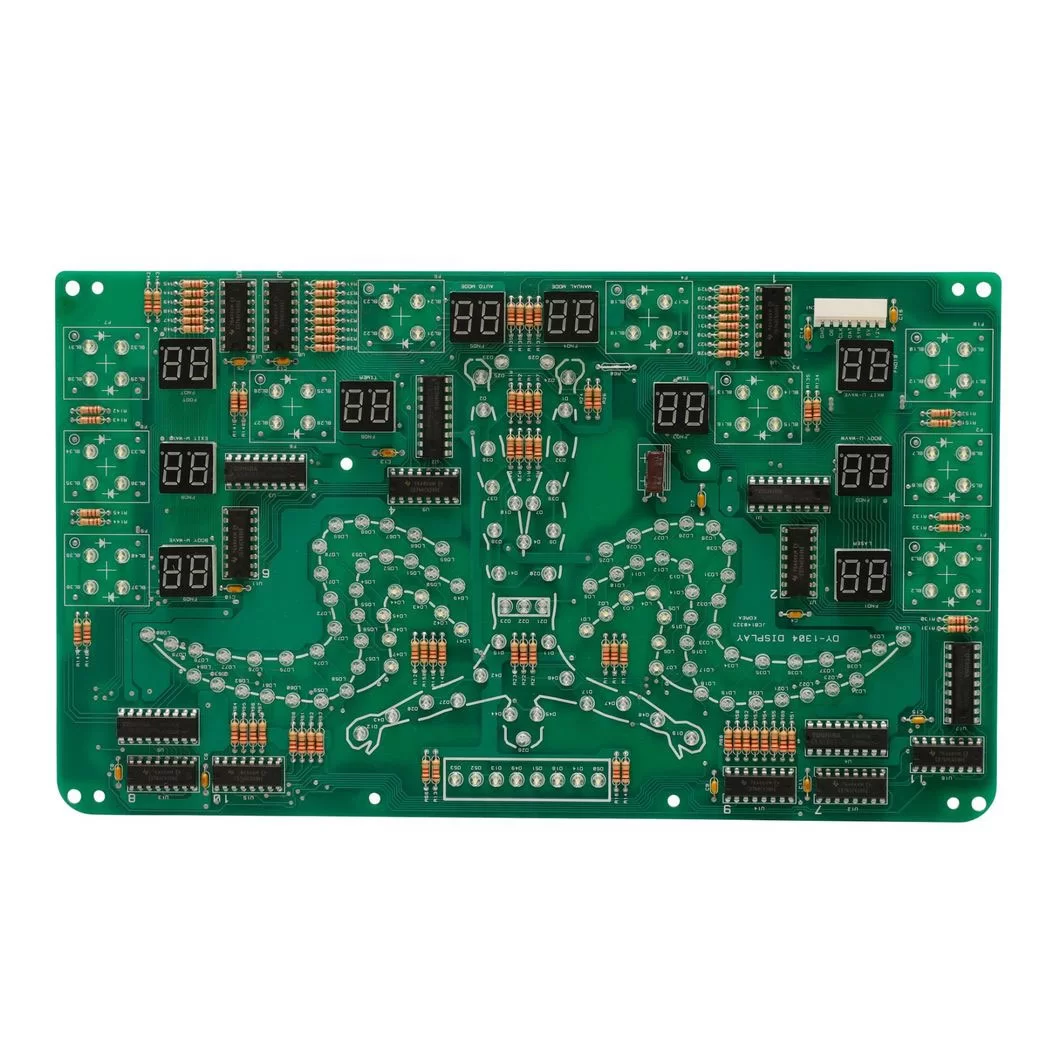
PCB type: Rigid PCB
Layer: single-sided
Base material: FR-4
Solder mask: Yellow
Silk screen: White
Surface treatment: Lead-free HASL

PCB type: Rigid PCB
Layer: Multi-layer
Base material: FR-4
Solder mask: Blue
Silk screen: White
Surface treatment: Lead-free HASL

PCB type: Rigid PCB
Layer: Multi-layer
Base material: FR-4
Solder mask: Green
Silk screen: White
Surface treatment: Lead-free HASL

Due to the specific nature of their industry applications, medical electronic PCBAs require extremely high performance standards.



